You may wonder:
- Is 100% pure gold possible?
- What is considered fine gold?
-
Why can’t you have pure gold?
The fact is…
Gold naturally cannot be 100% pure, it’s pretty much impossible to achieve. The ultra pure types of gold commercially available are 4 or 5 nines fine.
Yes, you heard that right.
Today, we’ll dive deep into this topic and discuss its natural state, how alloys influence its purity, and the cool science bits and the real reasons why it can’t it hit that perfect score.
Without further do, let’s get started.
Investing in precious metals? Join our #1 gold newsletter now. Use our code GSC20 to get 20% OFF any subscription plan!
Gold’s Natural State
So is 100% gold possible?
To become the precious metal star it is today, it involved exploding stars and some geology magic.
Imagine this: massive stars, millions of years ago, going about their business. Inside, they’re cooking up some fantastic elements, gold is one of them.
Then BOOM!
A supernova explosion sends these star-made goodies into space, sprinkling the universe with some golden stardust.
Fast forward a bit, and that stardust finds its way to our planet, making gold a part of our planet’s DNA.

Yep, it’s been hanging around since the Earth’s early gig days, chilling in veins, turning up as shiny nuggets, or sprinkling some glitter in alluvial deposits.
Talk about having a legacy, right?
But here’s the tricky part: Earth’s geology game is strong, and it loves mixing things up.
This means that geological processes like hydrothermal and magmatic activity are busy blending gold with other elements, which add to the metal’s impurity playlist.
Because of those elements, your gold jewelry has different shades.
Mix with silver and zinc, and it gets this pale or bright yellow appearance. Party with copper, and it rocks a reddish swagger.
This leads us to the next section…
The Influence of Alloys
You may wonder:
Why some gold jewelry is super sturdy, while others have that oh-so-romantic rosy glow? Or why does that family heirloom ring still shine bright while resisting those annoying scratches?
No doubt:
Pure gold is a showstopper, but here’s a little secret: on its own, it can be a bit of a softie.
So, over time, humans got crafty and started mixing it with other metals to give it that extra oomph.
The result?
A stronger, more resilient, and sometimes even funkier-colored gold.
By manually blending different elements, we get a vibrant hue effect.
Have a look at the caratage and the percentage of elements that make the yellow gold:
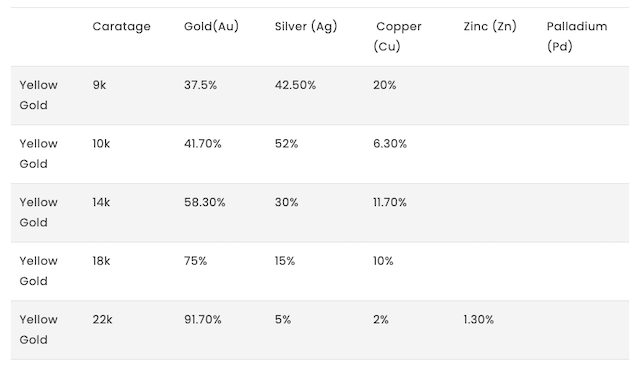
For example…
With the 18-karat gold, we see that it’s made of 75% gold and 25% other metals like silver and copper, which is the sweet spot between keeping that gold magic and having something strong enough for the everyday jewelry hustle.
You’ve also got rose gold, which brings romantic vibes with its warm hue thanks to a mix of gold, copper, and sometimes a dash of silver.
And for those who love that icy elegance, there’s white gold.
Mix it palladium, silver, or nickel, and you’ve got a metal that looks like the ritzy platinum but doesn’t burn a hole in your pocket.
But hey, these alloys aren’t just about looking good and aren’t in just a beauty industry.
You might find them in dental work or those tech gadgets you love (which often have gold alloy connectors with their top-notch electrical skills).
At this point you may wonder:
Does mixing gold with other metals make it less… gold?
Well, technically, yes. But think of it this way: It’s like giving gold a bit of a makeover to suit the occasion, whether it’s a glitzy party or hard work on technology.
Why Is Gold Never 100% Pure?
Let’s talk about about impurities and why can’t gold be 100% pure?
Now, the example we’ll use might sound a bit simplistic but will help understand the case better.
You see, when we get this precious yellow metal from the Earth, it’s like getting an ice cream sundae with lots of toppings.
The gold is your ice cream, but it often comes with sprinkles, nuts, and maybe some whipped cream in the form of other minerals.
And just like some of us think a sundae isn’t complete without those extras, this shiny metal naturally comes with its crew of other elements.
The whole process of getting it out is a bit like trying to get only the ice cream from a sundae without any toppings (tricky, right?).
Even after all that effort, we usually end up with one that has tiny bits of other stuff, like silver, copper, or zinc.
But here’s a twist:
Sometimes, these “add-ons” make it even more special. Like gold with some silver might have this super cool pale-yellow vibe, giving it its own unique charm different from the pure look.

Depending on mixed with it, our shiny metal can have different shades – from that classic gold shade to something paler or even a bit reddish or greenish.
And as we mentioned earlier, these alloy elements don’t just influence its look but also how it behaves – like how hard it is or its melting point.
Now…
“Do these extras make my yellow metal less valuable?”
Nope! They give each piece its own story and character.
It’s like having a handcrafted coffee with its unique flavor profile instead of just a regular cup of joe.
Purification Techniques
Here, we’ll use coffee as an example for simplicity.
Imagine having a cup of freshly brewed coffee, and you’re trying to remove every single coffee ground. Tough, right?
That’s how refining gold feels. It’s all about getting rid of the tiny bits we don’t want, making it shinier and more pure.
A long time ago, people used a method called fire assaying.
Think of it like melting a chocolate bar to remove the nuts–they’d melt gold and ditch the bits that didn’t belong.
Another cool trick was cupellation.
Here, they’d get the precious metal all cozy with lead, heat them up, and the lead would kind of kidnap all the unwanted bits, leaving just the gold behind.
But here’s the catch:
Some bits, like iridium or osmium, love clinging to gold so much that they’re super hard to separate. It’s like trying to pick out every single sprinkle from a donut–almost impossible and a lot of work!

In ancient times, people also tried this thing called “parting”.
Imagine dropping that sprinkle-filled donut into a liquid where only the sprinkles dissolve, leaving you with just the donut.
This process does something similar, dissolving only certain impurities and leaving the yellow metal behind.
Now, you might be wondering, “Why not go for 100% pure gold?”
This is because of how it is and the little bits that naturally tag along with it, getting absolute purity is like searching for a needle in a haystack.
But hey, it’s not all about getting the purest one. Sometimes, it’s the journey, the history, and the stories behind that piece that make it truly magical.
Theoretical Limits on Purity
So, those super stubborn impurities in gold are called noble impurities, and they just don’t want to budge.
Imagine trying to get rid of that one stubborn stain on your favorite shirt – no matter how much energy or fancy detergents you throw at it, it’s just there.
Hope you didn’t get tired of the everyday-like, simplistic examples today, but that’s kind of what it is like with these impurities.
Plus, trying to chase that 100% dream can really burn a hole in our pockets.
Sometimes, it just isn’t worth the extra cash or effort, especially when the super-pure gold doesn’t have many practical uses.
What is 999 purity gold?
The metal’s purity is measured in karats, and the golden standard (pun intended) is 24 karats, which has an ultra pure level of 99.99% (people in the know call it “four nines” fine gold).

Now, here’s the science bit:
This precious metal is made up of atoms.
Perfect purity would mean our piece has zero non-gold atoms. But nature’s got its own plans, and pure gold naturally has some other atoms mingling in there.
Plus, these so-called “impurities” actually have a job – they help stabilize the metal’s structure.
They are a small amount, but it makes a world of difference to the end product. Take out all the impurities, and this precious metal might just not act like the gold we know and love.
On top of that, these extra elements give it its charm, like a beautiful color, flexibility, and the ability to stay shiny without getting all tarnished.
So these things are the reason why gold can’t be 100% pure.
Investing in the U.S.? Our #1 Recommended Gold IRA Company
Over to you:
Do you agree with what was said here? Do you have anything else to add on why you can’t have pure gold?
Share your thoughts in the comments below!